Which Layer of the Fallopian Tube is Continuous With the Peritoneum
Diaa M. El-Mowafi - Zagagig University, Egypt
Fallopian Tube
Diaa M. EI-Mowafi, MD
Associate Professor, Department of Obstetrics & Gynecology, Benha Faculty of Medicine, Egypt
Researcher & Educator, Wayne State University, USA
Fellow, Geneva University, Switerland
Michael P. Diamond, MD
Professor, Department of Obstetrics & Gynecology, Wayne State University, USA
Director, Division of Reproductive Endocrinology & Infertility
- Embryology
- Anatomy
- Histology
- Physiology
- Tubal Disorders
- Tuboplasty
- Assisted Reproductive Techniques Involving the Tube
- Tubal Sterilization
- Methods for Tubal Investigations
GLOSSARY
- adhesions Scar fibrous tissue connecting sites at aberrant locations.
- ectopic pregnancy Pregnancy occurring outside of the uterine cavity, most commonly in the Fallopian tube.
- Fallopian tube A conduit allowing passage of egg from ovary to exit the abdominal cavity to enter the uterus; the site of fertilization in humans.
- falloposcopy Viewing the interior of the tubal lumen with a narrow fiber scope.
- tuboplasty Surgery on the tube, most commonly to restore tubal patency or reduce scarring to promote fertility. includes procedures such as neosalpingostomy and fimbrioplasty.
The Fallopian tube is essential for unassisted procreation, serving as a conduit for the ovulated oocyte (egg) to enter the uterine cavity following fertilization. Its functions are regulated both hormonally and by the nervous system, and it is the usual site for oocyte fertilization. Tubal function can be impaired by infection, surgery, adhesions, and other pathologic processes. Function of the tube can often be restored surgically or it can be bypassed through the use of in vitro fertilization of oocytes followed by intrauterine embryo transfer.
EMBRYOLOGY
Between the fifth and sixth week after oocyte fertilization, a longitudinal groove called Müller's groove arises from the coelomic epithelium on each side lateral to the mesonephric duct (Fig. 1). The edges of this groove fuse to form a canal called the Müllerian or paramesonephric duct. The Fallopian tubes develop from the cranial parts of the paramesonephric ducts, with their cranial ends remaining open connecting the duct with the coelomic (peritoneal) cavity and the caudal end communicating with the uterine cornua. Congenital anomalies of the tube include aplasia, in which the tube fails to form; hypoplasia, in which the tube is long, narrow, and tortuous; accessory ostia, and congenital diverticulae.
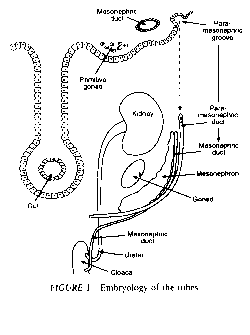
ANATOMY
The Fallopian tubes are paired, tubular, seromuscular organs whose course runs medially from the cornua of the uterus toward the ovary laterally. The tubes are situated in the upper margins of the broad ligaments between the round and utero ovarian ligaments (Fig. 2). Each tube is about 10 cm long with variations in length from 7 to 14 cm. The abdominal ostium is situated at the base of a funnel-shaped expansion of the tube, the infundibulum, the circumference of which is enhanced by irregular processes called fimbriae. The ovarian fimbria is longer and more deeply grooved than the others and is closely applied to the tubal pole of the ovary. Passing medially, the infundibulum opens into the thin-walled ampulla forming more than half the length of the tube and 1 or 2 cm in outer diameter; it is succeeded by the isthmus, a round and cord-like structure constituting the medial one-third of the tube and 0.5-1 cm in outer diameter. The interstitial or conual portion of the tube continues from the isthmus through the uterine wall to empty into the uterine cavity. This segment of the tube is about 1 cm in length and 1 mm in inner diameter.
HISTOLOGY
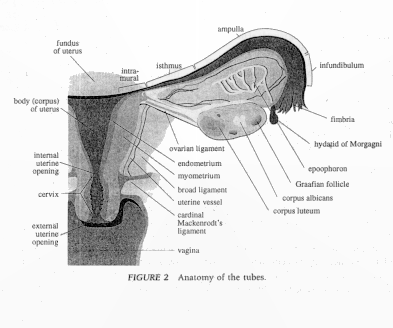
The tubal wall consists of three layers: the internal mucosa (endosalpinx), the intermediate muscular layer (myosalpinx), and the outer serosa, which is continuous with the peritoneum of the broad ligament and uterus, the upper margin of which is the mesosalpinx. The endosalpinx is thrown into longitudinal folds, called primary folds, increasing in number toward the fimbria and lined by columnar epithelium of three types: ciliated, secretory, and peg cells. In the ampullary and infundibular sections, secondary folds of the tubal mucosa also exist, markedly increasing the surface areas of these segments of the tube. The myosalpinx actually consists of an inner circular and an outer longitudinal layer to which a third layer is added in the interstitial portion of the tube.
The serosa of the tube is composed of an epithelial layer histologically indistinguishable from peritoneum elsewhere in the abdominal cavity.
PHYSIOLOGY
The tubes act as ducts for sperm, oocyte, and fertilized ovum transport, in addition to being the normal site of fertilization. These functions depend mainly on three factors: tubal motility, tubal cilia, and tubal fluid.
Tubal Motility
Peristaltic contraction of the smooth muscle fibers in the tubal wall allows the gametes (the sperm and egg) to be brought together, thus allowing fertilization and subsequent transport of the fertilized ovum from the normal site of fertilization in the ampulla to the normal site of implantation in the uterus. This movement is primarily regulated by three intrinsic systems: the estrogen-progesterone hormonal milieu, the adrenergic-nonadrenergic system, and prostaglandins.
Estrogens acting at a receptors stimulate tubal motility, whereas progesterone, which activates b receptors, inhibits tubal motility. Before ovulation, contractions are gentle, with some individual variations in rate and pattern. At ovulation, contractions become vigorous and the mesosalpinx contracts to bring the tube in more contact with the ovary while the fimbria contracts rhythmically to sweep over the ovarian surface. As progesterone level rises 4-6 days after ovulation, it inhibits tubal motility. This may lead to relaxation of the tubal musculature to allow passage of the ovum into the uterus by the action of the tubal cilia. The effects of estrogen and progesterone on oviductal motility and morphology is mediated through these steroids' receptors. The changes in receptors levels are critical in determining the functional state of the oviduct.
Adrenergic innervations are thought to be involved in regulations of tubal motility, particularly isthmic motility changes. During menstruation and the proliferative (preovulatory) phase, the human tube is very sensitive to a -adrenergic compounds such as norepinephrine. After ovulation and during the luteal phase, the response to norepinephrine is decreased and the inhibitory effect of b -adrenergic compounds is more evident. Estrogens potentiate the activation of a receptors, whereas progesterone potentiates the activation of b receptors. Activation of the receptors by raised progesterone level in the luteal phase leads to relaxation of the circular muscles; thus, the isthmic luminal diameter is increased and transisthmic passage of the fertilized ovum is facilitated.
Although there is a controversy regarding the role of prostaglandins in the regulation of spontaneous tubal motility, it has been found that prostaglandin F2 a (PGF2 a ) stimulates whereas PGE1 and PGE2 inhibit Fallopian tube contractions. Contrary to their differential activity on tubal motility, all three natural prostaglandins (PGF2, PGE1, and PGE2) stimulate ciliary activity in vitro.
In summary, the initial rise in progesterone after ovulations causes b - mediated contractions of the two inner layers of the uterotubal junction, thus causing tubal locking of the ovum. After a few days, sensitivity of the muscles to adrenergic stimulation diminishes, whereas other factors, such as prostaglandins, dominate leading to relaxation of the uterotubal junction and release of the fertilized ovum into the uterine cavity.
Tubal Cilia
There are fewer ciliated cells in the isthmus than in the ampullary portion of the tube, whereas they are most prominent in the fimbriated infundibulum. Ciliation and deciliation is a continuous process throughout the menstrual cycle. Ciliation is maximum in the periovulatory period, particularly in the fimbria. Estrogen enhances the process of ciliation, whereas progesterone inhibits it, so significant deciliation occurs in atrophic postmenopausal tube.
Ciliary activity is responsible for the pickup of ova by, the fimbrial ostium and movement through the ampulla, as well as the distribution of the tubal fluid which supports gamete maturation and fertilization and facilitates gamete and embryo transport. The close approximation between the ovary and fimbria is likely to be important for ovum pickup, although, transperitoneal migration has been reported. The importance of ciliary activity is affirmed by the tubal dysfunction seen in associations with the deciliation of salpingitis. Questions are raised, however, in women suffering from Kartagener's syndrome, which is the immotile cilia syndrome, when the women are still fertile.
Tubal Fluid
Tubal fluid is rich in mucoproteins, electrolytes, and enzymes. This fluid is abundant in midcycle when gametes or embryos are present and may play an important role during fertilization and early cleavage. Fluid in the tubes is believed to be formed by (i) selective transudation from the blood and (ii) active secretion from the epithelial lining. The rate of fluid accumulation is 1-3 ml/24 hr and the rate of production is increased significantly around the time of ovulation.
TUBAL DISORDERS
Pelvic inflammatory Disease
Pelvic inflammatory disease (PID) is inflammation of the upper genital tract characterized primarily by salpingitis. The disorder may coexist with endometritis or oophoritis, may spread as peritonitis, and may extend along the paracolic gutters to the liver to cause the Fitz-Hugh-Curtis syndrome.
The cervical barrier plays a crucial role in preventing the ascent of vaginal organisms to the upper genital tract. This barrier may be compromised after miscarriage, delivery, cervical surgery such as amputation, dilatation, and cauterization, or at the time of an intrauterine device insertion. The causative organism in most initial cases is Chlamydia trachomatis; Neisseria gonorrhoeae, Mycoplasma hominis, Ureaplasma uralytica, and Actinomyces israelii have also been found to be a cause in some cases. In subsequent episodes of PID, other aerobic and anaerobic organisms may be the causative agent to the pelvic infection. The infection usually starts as an asymptomatic cervicitis. If the natural barriers such as the narrow endocervical canal, the downward flow of mucus, the presence of antibacterial lysozymes, and the production of specific local IgA fall, the infection may spread to the endometrium. The monthly shedding of the endometrium is another protection against infection, aided by the mechanical effect of the uterotubal junctions and the ciliary activities of the Fallopian tubes which creates a downward flow of tubal fluid. Therefore, not all the organisms that reach the endometrial cavity necessarily spread to the Fallopian tubes. Nonetheless, the open tubal lumen allows spread of infection to the peritoneum, causing salpingooophritis and peritonitis. Consequences of PID include blockage of the tube usually either proximately at the site of insertion into the uterus or distally causing a hydrosalpinx with partial or complete distal obstruction. Less commonly, a midtubal segment of the tube may become occluded. Other sequelae may include pyosalpinx, tubal or tuboovarian abscess, and peritubal adhesions. The long-term consequences of PID include recurrent PID in almost 25% of cases after one episode of salpingitis, chronic pelvic or abdominal pain in one of every five affected patients, tuboovarian abscess in about 34% of hospitalized patients, Fits-HughCurtis syndrome, deep dyspareunia in two of every five patients, and menstrual disturbances in four of every five patients. Additionally, the risk of ectopic pregnancy increases seven times that of control sub,jects,. The risk of subsequent infertility is approximately 12% after one episode of PID, 35% after two episodes, and 75% after three or more episodes.
Ectopic Pregnancy
The incidence of ectopic pregnancy has significantly increased during the past two decades-more than fourfold from 4.8 to 20.9 per 1000 live births. However, the mortality rate due to ectopic decreased significantly during the same period from 53.2 to 5.3 deaths per 10,000 cases, perhaps due to more awarenes by patients and health care providers. However, ectopic pregnancy remains the second leading cause of maternal mortality in the United States.
Ectopic pregnancy results from a delay in the passage of the fertilized ovum through Fallopian tube. This delay can result from anatomical abnormalities of the tubes, such as constriction and false passage formation (e.g. diverticulum), or from tubal dysfunction as altered contractility or abnormal ciliary activity. Tubal anatomy and function can both be altered by either tubal surgery or prior PID. These are often present together in the same individual, The surgical procedures predisposed to ectopic pregnancy include salpingolysis and ovariolysis, fimbrioplasty, neosalpingostomy, and tubal anastomosis. In fact, our success of microsurgery may have allowed patency of tubes to be restored, only to predispose these patients to ectopics. Approximately 95% of ectopics occur in the tubes, in which most of them are located in its distal parts, particularly the ampulla. The other 5% of cases occur in the ovaries a rudimentary horn of bicornuate uterus, broad ligaments, peritoneum, and cervix.
Treatment of tubal pregnancy may be expectant, medical, or surgical. Asymptomatic tubal pregnancies with low and falling b -human chorionic gonadotropin (hCG) levels 48 hr apart may be treated expectantly in reliable patients with readily available access to the hospital. Such tubal gestations frequently resolve spontaneously, but treatment has limitations and a relatively high failure rate. Medical therapy may be systemic or local. Intramuscular methotrexate at a dosage of 1 mg/kg per day on alternative days with cirtovorum factor rescue at a dosage of 0.1 mg/kg for a total period of 8 days or a single-dose methotrexate (1 mg/kg), is the most commonly used protocol. Failure is more likely to occur if b -hCG levels exceed 6500 mIU/ml or if fetal heart motion is identified on ultrasonography. Local injection of methotrexate, PGF2a or hypertonic glucose solution into the tubal pregnancy under laparoscopic visualization appears to be effective in selected patients. Local medical treatment is also plausible, with the drug injected transvaginally under ultrasonographic control and local anesthesia.
Surgical treatment can be achieved via laparotomy or laparoscopy. Through laparotomy, one of the following procedures may be performed:
- Salpingostomy: Used to treat unruptured ampullary gestations. A linear incision is performed in the antimesentric border of the tube and the ectopic gestation is expressed through it. The edges of the incision can be cauterized if bleeding occurs, but this is otherwise usually not approximated by sutures. In the ampullary segment of the tube, these will usually heal by secondary intention without fistula formation or tubal obstruction. In part, this healing profile may stem from tlic frequent localization of ampullary ectopics not in the tubal lumen but rather in the potential space between the scrosa and muscularis. A salpingotomy varies slightly from a salpingostomy in that there is suturing of the incision edges by sutures. Often, salpingostomy may be preferred because sutures may increase the incidence of postoperative adhesions.
- Salpingectomy: Performed in a patient in whom a tube is thought to be nonsalvagable or a patient who does not desire future conception. It may be partial or complete. The removal of the ipsilateral ovary is not recommended, as was once the common practice, unless the ovary is involved with the ectopic or other ovarian pathology exists.
- Segmental resection and anastomosis: Used most commonly for unruptured ectopic in the isthmus of the tubes since this part is narrow, and if salpingostomy is performed proximal tubal obstruction and subsequent ectopic may occur. Anastomosis is often difficult during the unscheduled times surgical treatment of ectopics occur because operating room staff may be unfamiliar with microsurgical equipments. Additionally, edema and vascularity of the tube may be increased at this time. An alternative is to perform resection, with reanastomosis at a later time. If this option is chosen, the woman must be counseled that she can develop a repeat ectopic in the blindly ending distal segment. A third option is to perform a linear salpingostomy, thereby accepting the increased risk of fistula formation or occlusion but avoiding a repeat surgery in women in whom the tube heals without complication.
- Fimbrial evacuation: The distally implanted tubal pregnancy is evacuated by "milking" or "suction" through the fimbrial end. The drawback of this method, is that the incidence of recurrent ectopic is double that of salpingostomy and bleeding from the implantation site may continue. However, if the eccyesis is already extruding from the fimbriated end of the tube, grasping the products of conception is a reasonable alternative.
Through laparoscopy, one of the following procedures may be performed if the patient is hemodynamically stable:
- Linear salpingostomy: After the injection of vasopressin in the mesosalpinx, an incision is made along the antimesentric border of the ectopic with the CO2 laser, the argon laser, or by electrocoagulation. The ectopic gestation and the surrounding clot often extrude from the incision. The tissue is then grasped and removed through one of the second puncture probes. An irrigator can be placed into the incision, and irrigation under pressure may dislodge the eccyesis. Bleeding points are secured by bipolar diathermy and salpingostomy incision is left unsutured to heal by second intention.
- Segmental resection: The bipolar electrocautery forceps is applied distal to the target segment. The tube, mesosalpinx, and blood vessels are fulgurated in successive steps toward the uterus. The specimen is then sharply resected with laparoscopic scissors.
- Salpingectomy: The tube, together with the adjacent mesosalpinx, is grasped, electrocoagulated, and divided at the level of the uterotubal junction. The tuboovarian ligament is then grasped, electrocoagulated, and divided. The mesosalpinx is then electrocoagulated and divided until complete excision of the tube is accomplished.
Salpingitis Isthmica Nodosa
Salpingitis isthmica nodosa (SIN) is a disease process characterized by nodular thickenings in the intramural (interstitial) and isthmical part of the tubes.
It is common around the age of 35 and more common among African Americans. The exact cause of the disease is unknown, but the inflammatory process due to tuberculosis, gonorrhea, or bilharziasis is the most accepted explanation. The condition is bilateral in about 35% of cases; the nodules vary from a few millimeters to 2.5 cm in diameter. These are firm with a smooth surface, giving a beaded appearance. Microscopically, these are gland-like spaces scattered throughout the myosalpinx with hyperplasia and hypertophy of muscle fibers. The surrounding stroma is infiltrated with plasma cells or eosinophils. This condition has the sequence of ectopic pregnancy and infertility due to occlusion at the isthmic-ampullary junction.
Neoplasms of the Fallopian Tubes
Although the Fallopian tubes are a common site of metastatic spread, primary tumors are rare. The benign tubal neoplasms include adenomatoid tumors, leiomyoma, teratomas, fibroma, fibroadenoma, papilloma, lipoma, hemangioma, lymphangioma, mesothelioma, and mesonephroma. The malignant tumors are either secondary or primary as adenocarcinoma, sarcoma, and choriocarcinoma. Tubal carcinoma is the rarest genital cancer, represented about 0.3% of all genital malignancy, and the age of incidence is 40-60 years. It is commonly mistaken with ovarian carcinoma but tubal carcinoma is characterized by being mainly in the tube and shows a papillary pattern; microscopically the tubal mucosa should be involved and the transition between benign and malignant tubal epithelium is demonstrated. The risk factors for tubal carcinoma are infertility, which was found in up to 70% of cases, and chronic salpingitis, which may itself be the cause of infertility. The clinical presentation which may suggest tubal cancer is the triad of pelvic pain, serosanguinous vaginal discharge escaping continuously or in gushes (hydrops tubae profluens), and pelvic mass.
TUBOPLASTY
The term "tuboplasty" refers to a broad group of surgical procedures involved with the surgical correction of' tubal disease, which may contribute to infertility. Most of these procedures can be performed at laparoscopy as well as laparotormy. Regardless of how the procedures are performed, the principles of microsurgery should be applied to the extent possible, including the use of magnification, fine caliber microsurgical instruments, meticulous hemiostasis, minimization of tissue handling, prevention of tissue desiccation, avoidance of introduction of foreign bodies, and use of fine suture material of low tissue reactivity. In contrast to prior inclusion of precise reapproximation of all tissue planes, we believe it may be better to apply this "tenet" in situations in which the surgeon wishes to protect or isolate underlying tissue. The following are types of tuboplasty:
- Salpingolysis: Lysing adhesions from the tube to adjacent surfaces such as the ovary, uterus, bowel, and peritoneal surfaces.
- Resection and anastomosis: Performed for reversal of sterilization procedures or treatment of' pathologic tubal obstructions.
- Neosalpingostomy: Creation of a new tubal ostium whether terminal, ampullary, or isthmic. The tube may be a hydrosalpinx but this is not required. In patients with a hydrosalpinx, the likelihood of conceiving following surgical correction will be related to the size of the hydrosalpinx, the thickness of the tubal wall, and perhaps most important, the extent of damage to the tubal mucosa as assessed by loss of tubal folds and appearance of the fimbria.
- Fimbrioplasty: Operation on the distal end of the tube, including reconstruction of existent fimbria by ostial stretching, deagglutination, lysis of perifimbrial adhesions, and incisions through the tubal wall to recover fimbriae. A fimbrioplasty is distinguished from a neosalpingostomy in that a fimbrioplasty has at least a partial ostium remaining. Because of the diversity in tubal damage which can be treated by a "fimbrioplasty" (from a filmy adhesion to the fimbria to a large hydrosalpinx with a pinpoint opening), success following treatment is extremely variable.
- Tubouterine implantation: Suitable for cornual obstruction unamenable to anastomosis in which the blocked segment is excised and tube is reimplanted into the uterine cornu or fundus. This procedure is currently infrequently performed because of low success rates.
- Tubal cannulation: Proximal tubal blockage as assessed by hysterosalpingogram, chromopertubation, or hysteroscopy may represent true obstruction or the presence of an occluding agent such as a mucus plug. Transcervical probing of the proximal Fallopian tube with a special fine guide wire under fluoroscopic guidance or through hysteroscopic catheterization may be effective in dislodging debris or breaking up fine adhesions which cause tubal occlusion and result in infertility. In the past, these patients would have needed laparotomy with tubal microsurgery or in vitro fertilization. A 58% pregnancy rate was achieved by this procedure in carefully selected patients in whom proximal tubal obstruction was identified to be the primary or sole cause of infertility.
ASSISTED REPRODUCTIVE TECHNIQUES INVOLVING THE TUBE
Assisted reproductive technologies (ART) are utilized as a means of helping infertile couples to conceive. They all involve recovery (aspiration) of oocytes from the ovary (usually under transvaginal ultrasound guidance). In in vitro fertilization (IVF), the oocytes are incubated with spermatozoa (the male gamete) and then evaluated approximately 18 hr later to determine whether fertilization has occurred. The zygote is then cultured an additional 24-48 hr, during which time embryo cellular division occurs, followed by transcervical transfer of embryos into the uterine cavity. Alternative methods of ART are to transfer the gametes or zygotes into the Fallopian tubes using the following procedures:
- Gamete intra-Fallopian transfer: Sperm and a limited number of oocytes (usually two to four) are loaded into a special catheter with a culture medium, and the gametes are delivered through the laparoscope or a transcervical catheter into the Fallopian tube.
- Zygote intra-Fallopian transfer: The zygote is transferred into the ampulary part of the tube approximately 18-24 hr after fertilization between the male and female gametes in vitro.
TUBAL STERILIZATION
The tubes, as a target to induce female sterilization, can be approached via different routes, namely; laparotomy, minilaparotomy, laparoscopy, colpotomy, and hysteroscopy.
Laparotomy: Many procedures have been used; the following are the most common:
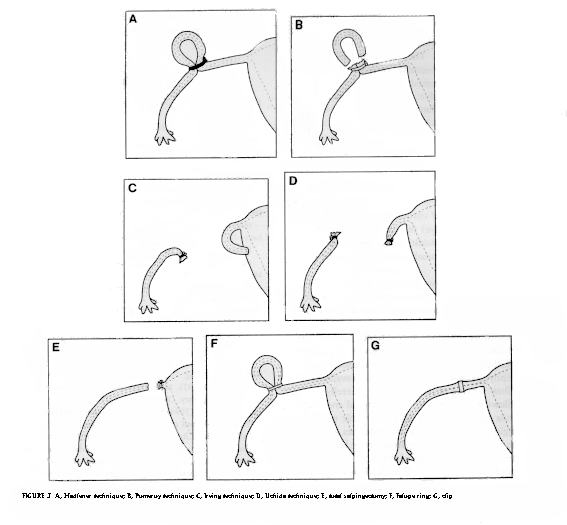
- Simple ligation: Ligation of the tube in continuity.
- Madlener (1919): Crushing and ligation of a loop of the tube (Fig. 3A).
- Pomeroy (1929): Ligature around a loop of the tube and excision (Fig. 3B).
- Irving (1924): Double ligature, excision of a tubal segment, and concealing of the proximal end, which is buried in the myometrium, and the distal end, which is buried in the broad ligament (Fig. 3C) '
- Uchida (1975): Tubal serosa is stripped from muscular coat, tubal segment is excised, and proximal end is ligated and buried in the broad ligament (Fig. 3D).
- Kroener (1969): Fimbriectomy, ligation of distal end of tube, and mesosalpinx and excision of fimbriated end.
- Parkland: Double ligature and removal of a segment in between.
- Salpingectomy: Removal of both tubes (Fig. 3E).
- Cornual resection: Removal of theinterstitial portion of the tube together with a wedge of myometrium.
Minilaparotomy: A small transverse abdominal incision 2 or 3 cm in length through which the Pomeroy, Parkland, or Kroener techniques can be utilized.
Laparoscopy :
- Electrosurgery: i- monopolar and ii- bipolar; electrical coagulation of adjacent segments of the tubes with or without transaction of the tubes.
- Fallope ring: A Loop of tube is drawn into an applicator tube; a silastic ring is placed around the tubal loop (Fig. 3F).
- Clips (e.g., Hulka or Filshie): Plastic crushing clips are placed across the tube (Fig. 3G).
Colpotomy: Whether anterior or posterior, through which one of the previously mentioned procedures done through laparotomy can be accomplished. This option is currently infrequently performed.
Hysteroscopy: Experimental approaches have been attempted by the following:
- Electrocoagulation
- Chemical scarification
- Intratubal plugging devices (ITD)
- Uterotubal junction devices: Placed into the uterotubal.junction and held in place by lateral "wings" at the distal tip and a loop-size extension of the proximal part. The uterotubal junction is not occluded.
- Blocking uterotubal junction devices: Occlude the intramural part of the tube. However, this part of the tube is a muscular structure and expulsion or pain are frequent drawbacks. A special type of these devices is the P-block developed by Brundin. The P-block consists of a nylon skeleton with a 4-mm long hydrogel body and two nylon, anchoring wings. After insertion, the hydrogel expands and occludes the intramural part of the tube.
- Intratubal formed-in-place silicone devices (Ovabloc ITDs): Ovabloc ITDs are formed in situ after the intratubal administration of liquid medical-grade silicone rubber mixed with catalyst in a mixer-dispenser. Radiopaque spherical silver powder is added to the silicone so that the ITD can be identified on X ray.
The failure rate of commonly utilized methods of tubal sterilization is variable from one method to the other, but generally it is approximately 0.3-1%; this may be due to misidentification of the tubes or recanalization. The success rate of reversal of the sterilization is dependent on the method that was used for sterilization; the less damage and shorter damage of the tubal segment the higher the success rate. It is claimed that the pregnancy rate after reversal of sterilization can approach 70%, but this is dependent on multiple factors including the woman's age, the location of the anastomosis, the final tubal length, and coexistent, pelvic pathology.
METHODS FOR TUBAL INVESTIGATIONS
The patency, course, and function of the tubes can be in part assessed by the following methods:
Hysterosalpingography: A radiopaque substance, such as lipoidal, or a water-soluble medium, such as urograffin, is injected into the uterus through a cervical canula. Imaging of the uterine cavity and tubes is obtained by flouroscopy and X ray. The course and patency of the tube and potentially some peritubal adhesions can be assessed, the latter by intraperitoneal pooling of dye. Recently, sonography during intrauterine fluid instillation has been utilized to assess the uterine cavity. This method may also be applicable to assess tubal patency reliably, particularly as contrast agents which allow better visualization of tubal flow become more available.
Laparoscopy: Allows evaluation of the external tubal surface and peritubal pelvic adhesions, and by the addition of chromopertubation (the passage of colored dye) the patency of the tubes can be assessed. Additionally, the presence of a hydrosalpinx or tubal saccluations can be identified.
Salpingoscopy: Allows a panoramic view of the entire tubal mucosa, from the fimbria to the isthmus, and evaluation of intraluminal pathology. It may have a therapeutic role as well in lysing intratubal adhesions and/or treating other tubal pathology. Two types have been utilized in the past: the rigid and flexible salpingoscoies. Currently, narrow fibers are becoming available to allow viewing of the entire tube, thereby allowing examination of the cornual and isthmus segments which were not visible using rigid scopes. Currently, these devices are used in conjunction with laparoscopy or laparotomy to allow manipulation of the tube to facilitate passage of the scopes. If in the future it is possible to view the tubal lumen following transcervical passage without operative intervention, the clinical diagnostic value would increase dramatically.
Bibliography
- Diamond, M. P. (1988). Surgical aspects of infertility. In Gynecology and Obstetrics (j. W. Sciarra, Ed.), pp. 1-23. Harper & Row, Philadelphia.
- El-Mowafi, D. M. (1995). Gynecology Simplified. Dar El wafaa, Egypt.
- Hershlag, A., Diamond, M. P., and DeCherney, A. H. (1989). Tubal physiology: An appraisal. J. Gynecol. Surg. 5, 2-25. Hershlag, A., Scifer, D. B., Carcangiu, M. L., Patton, D. I., Diamond, M. P., and DeCherney, A. H. (1991). Salpingoscopy: Light microscopic and electron microscopic correlations. Obstet. Gynecol. 88, 399-405. jansen, R. P. S. (1984). Endocrine response in the fallopian tube. Endocr. Rev. 2, 525-549.
- Lavyl G., Diamond, M. I'., and DeCherney, A. H. (1987). Ectopic pregnancy: Its relationship to tubal reconstructive surgery. Fertil. Steril. 47, 543-556.
- Marshall, J. M. (1 98 1). Effects of ovarian steroids and pregnancy on adrenergic nerves of uterus and oviduct. Ain. J. Physiol. 240, 165-174.
- Penzias, A. S., Cutillann, J. N., and Diamond, M. P. (1993). Laparoscopic management of ectopic pregnancy. In Extrauterine Pregnancy: Clinical Diagnosis and Management (T. Stovall and F. King, Eds.), pp. 231-248. McGraw-Hill, New York.
- Stoyall, T. G., Ling, F. W., Gray, L. A., Carson, S. A., and Buster,j. E. (1991). Methotrexate treatment of unruptured ectopic pregnancy. Obstet. Gynecol. 77, 749-753.
- Thyrmond, A. 5. (1994). Transcervical tubal cannulation in the diagnosis and treatment of tubal obstruction. In The Fallopian Tube Clinical and Surgical Aspects 0. G. Grudxinskas et at., Eds.), pp. 151-156. Springer-Verlag, New York. Westrom, L., losif, 5., Svensson, L., and Mardh, P.-H. (1979). Infertility after acute salpingitis: Results of treatment with different antibiotics. Curr. Ther. Res. 26, 752-764.
Source: https://www.gfmer.ch/International_activities_En/El_Mowafi/Fallopian_tube.htm
0 Response to "Which Layer of the Fallopian Tube is Continuous With the Peritoneum"
Post a Comment